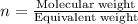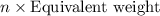Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Which information must be known about a compound to find the molecular formula from the empirical fo...
Questions



History, 13.11.2020 20:30



Chemistry, 13.11.2020 20:30




Chemistry, 13.11.2020 20:30


Mathematics, 13.11.2020 20:30


Mathematics, 13.11.2020 20:30

Biology, 13.11.2020 20:30

Social Studies, 13.11.2020 20:30

Arts, 13.11.2020 20:30

Mathematics, 13.11.2020 20:30