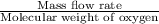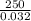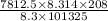Chemistry, 03.10.2019 02:00 alannaswitzer
Astream of oxygen at -65°c and 8.3 atm flows at a rate of 250 kg/h. use the srk equation of state to estimate the volumetric flow rate (l/hr) of this stream. (see example 5.3-3.) the ideal gas equation of state is an approximation. under which conditions, it is suggested that the ideal gas equation be used for? select one: o a. temperatures above about 0°c and pressures below about 1 atm o b. temperatures below about 0°c and pressures below about 1 atm c. temperatures above about 0°c and pressures above about 1 atm d. under any condition e. standard condition of 25°c and 1 atm o o

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

You know the right answer?
Astream of oxygen at -65°c and 8.3 atm flows at a rate of 250 kg/h. use the srk equation of state to...
Questions




Biology, 01.07.2019 22:30




History, 01.07.2019 22:30






Computers and Technology, 01.07.2019 22:30




Computers and Technology, 01.07.2019 22:30

Mathematics, 01.07.2019 22:30