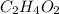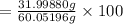Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

You know the right answer?
Acetic acid (hc2h3o2) is the active ingredient in vinegar. calculate the mass percent composition of...
Questions




Mathematics, 09.02.2021 05:00

History, 09.02.2021 05:00



Mathematics, 09.02.2021 05:00




Mathematics, 09.02.2021 05:00

Biology, 09.02.2021 05:00






Biology, 09.02.2021 05:00