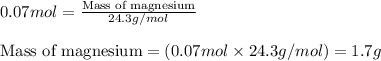Magnesium (used in the manufacture of light alloys) reacts with iron(iii) chloride to form magnesium chloride and iron. 3mg(s) + 2fecl₃(s) → 3mgcl₂(s) + 2fe(s)a mixture of 41.0 g of magnesium ( = 24.31 g/mol) and 175 g of iron(iii) chloride ( = 162.2 g/mol) is allowed to react. identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete. a) limiting reactant is mg: 67g of fecl₃ remains. b) limiting reactant is mg: 134 g fecl₃ remains. c) limiting reactant is mg: 104 g fecl₃ remains. d) limiting reactant is fecl₃: 1.7 g of mg remans. e) limiting reactant is fecl₃: 87.2 g of mg remains.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

You know the right answer?
Magnesium (used in the manufacture of light alloys) reacts with iron(iii) chloride to form magnesium...
Questions



Mathematics, 03.10.2019 06:40

Health, 03.10.2019 06:40



Mathematics, 03.10.2019 06:40

Mathematics, 03.10.2019 06:40



Social Studies, 03.10.2019 06:40



English, 03.10.2019 06:40






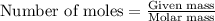 .....(1)
.....(1)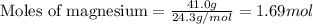
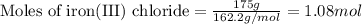
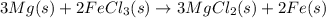
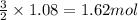 of magnesium
of magnesium