
Chemistry, 06.10.2019 04:00 zwbaby3693
At 298 k, the equilibrium concentration of o2 is 1.6 x 10-2 m, and the equilibrium concentration of o3 is 2.86 x 10-28 m. what is the equilibrium constant of the reaction at this temperature?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
At 298 k, the equilibrium concentration of o2 is 1.6 x 10-2 m, and the equilibrium concentration of...
Questions

Spanish, 30.10.2021 01:10


Social Studies, 30.10.2021 01:10


Mathematics, 30.10.2021 01:10

Mathematics, 30.10.2021 01:10

Computers and Technology, 30.10.2021 01:10


Mathematics, 30.10.2021 01:20

Chemistry, 30.10.2021 01:20

Mathematics, 30.10.2021 01:20


Mathematics, 30.10.2021 01:20


Business, 30.10.2021 01:20

Biology, 30.10.2021 01:20

Mathematics, 30.10.2021 01:20


Mathematics, 30.10.2021 01:20

English, 30.10.2021 01:20


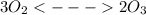
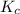 is the equilibrium constant and is defined as products concentration over reactant concentration and the coefficient is raised to its power. Thus we have the
is the equilibrium constant and is defined as products concentration over reactant concentration and the coefficient is raised to its power. Thus we have the ![K_c = \frac {[Products concentration]}{[Reactants concentration]}](/tpl/images/0291/9821/c00b5.png)
![K_c =\frac {[O_3 ]^2}{[O_2 ]^3}](/tpl/images/0291/9821/20678.png)
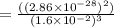
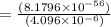
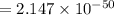 (Answer)
(Answer)

