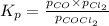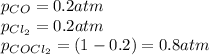Cocl2(g) decomposes according to the equation above. when pure cocl2(g) is injected into a rigid, previously evacuated flask at 690 k, the pressure in the flask is initially 1.0 atm. after the reaction reaches equilibrium at 690 k, the total pressure in the flask is 1.2 atm. what is the value of kp for the reaction at 690 k?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
Cocl2(g) decomposes according to the equation above. when pure cocl2(g) is injected into a rigid, pr...
Questions


Mathematics, 10.07.2019 06:50

Geography, 10.07.2019 06:50



English, 10.07.2019 06:50

History, 10.07.2019 06:50

Health, 10.07.2019 06:50

Mathematics, 10.07.2019 06:50


History, 10.07.2019 06:50

Mathematics, 10.07.2019 06:50

Mathematics, 10.07.2019 06:50



Geography, 10.07.2019 06:50


English, 10.07.2019 06:50


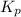 for the reaction at 690 K is 0.05
for the reaction at 690 K is 0.05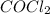 = 1.0 atm
= 1.0 atm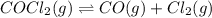
![[(1 - x) + x+ x]=1.2\\\\x=0.2atm](/tpl/images/0292/9648/696d1.png)