
Chemistry, 06.10.2019 09:02 oliviaschmitt0
The blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with an acidic potassium dichromate solution, resulting in the production of cr3+(aq) and carbon dioxide. the reaction can be monitored because the dichromate ion (cr2o72−) is orange in solution, and the cr3+ ion is green. the unbalanced redox equation is the following. cr2o72−(aq) + c2h5oh(aq) → cr3+(aq) + co2(g) if 31.91 ml of 0.0613 m potassium dichromate solution is required to titrate 33.8 g of blood plasma, determine the mass percent of alcohol in the blood.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2



Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1
You know the right answer?
The blood alcohol (c2h5oh) level can be determined by titrating a sample of blood plasma with an aci...
Questions




Mathematics, 04.03.2020 05:20

Mathematics, 04.03.2020 05:20



Spanish, 04.03.2020 05:21

Computers and Technology, 04.03.2020 05:21



Social Studies, 04.03.2020 05:21

Business, 04.03.2020 05:21

Social Studies, 04.03.2020 05:22

Biology, 04.03.2020 05:22

English, 04.03.2020 05:22


Mathematics, 04.03.2020 05:23


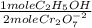 = 9,780x10⁻⁴ moles of C₂H₅OH
= 9,780x10⁻⁴ moles of C₂H₅OH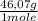 = 0,04506 g of C₂H₅OH
= 0,04506 g of C₂H₅OH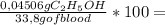 0,1333% of alcohol in the blood
0,1333% of alcohol in the blood

