
Chemistry, 05.10.2019 04:10 shels10tay
Be sure to answer all parts. write the balanced equations corresponding to the following rate expressions: a) rate = − 1 3 δ[ch4] δt = − 1 2 δ[h2o] δt = − δ[co2] δt = 1 4 δ[ch3oh] δt (click in the answer box to activate the palette. do not include states of matter.) b) rate = − 1 2 δ[n2o5] δt = 1 2 δ[n2] δt = 1 5 δ[o2] δt (click in the answer box to activate the palette. do not include states of matter.) c) rate = − 1 2 δ[h2] δt = − 1 2 δ[co2] δt = − δ[o2] δt = 1 2 δ[h2co3] δt (click in the answer box to activate the palette. do not include states of matter.)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1


Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
Be sure to answer all parts. write the balanced equations corresponding to the following rate expres...
Questions



Mathematics, 22.07.2019 04:00


Chemistry, 22.07.2019 04:00


English, 22.07.2019 04:00



History, 22.07.2019 04:00



World Languages, 22.07.2019 04:00




History, 22.07.2019 04:00

Mathematics, 22.07.2019 04:00


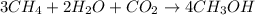
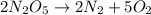
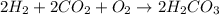
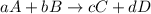
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0288/1459/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0288/1459/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0288/1459/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0288/1459/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0288/1459/d4b94.png)
![Rate=-\frac{1}{3}\frac{d[CH_4]}{dt}=-\frac{1}{2}\frac{d[H_2O]}{dt}=-\frac{d[CO_2]}{dt}=+\frac{1}{4}\frac{d[CH_3OH]}{dt}](/tpl/images/0288/1459/a1c90.png)
![Rate=-\frac{1}{2}\frac{d[N_2O_5]}{dt}=+\frac{1}{2}\frac{d[N_2]}{dt}=+\frac{1}{5}\frac{d[O_2]}{dt}](/tpl/images/0288/1459/697db.png)
![Rate=-\frac{1}{2}\frac{d[H_2]}{dt}=-\frac{1}{2}\frac{d[CO_2]}{dt}=-\frac{d[O_2]}{dt}=+\frac{1}{2}\frac{d[H_2CO_3]}{dt}](/tpl/images/0288/1459/13070.png)


