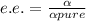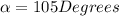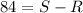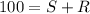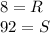Chemistry, 05.10.2019 04:20 AaronMicrosoft15
Ibuprofen is a drug used to manage mild pain, fever, and inflammation. it is a chiral drug, and only the s enantiomer, whose specific rotation is 125.0°, is effective. the r enantiomer exhibits no biological activity. if a particular process is capable of producing ibuprofen in 84% enantiomeric excess of the s enantiomer, then what is the specific rotation of that mixture? what is the percentage s enantiomer in that mixture?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
Ibuprofen is a drug used to manage mild pain, fever, and inflammation. it is a chiral drug, and only...
Questions


English, 01.02.2021 17:10


English, 01.02.2021 17:10



History, 01.02.2021 17:10



Mathematics, 01.02.2021 17:10




English, 01.02.2021 17:10

Arts, 01.02.2021 17:10

Social Studies, 01.02.2021 17:10


Mathematics, 01.02.2021 17:10