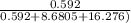Chemistry, 05.10.2019 04:20 dexterwilliams161
4. a gas containing equal parts methane, ethane and ammonia flows at a constant rate through a laboratory water- based absorption unit, which absorbs 96% of the ammonia and retains all the water. no methane or ethane is absorbed and no water evaporates. initially there was 5 l of water in the absorber, at the end of 4 hours the liquid mass is 5.25 kg. calculate the molar flowrate of the gas stream into the absorber, and the mole fraction of ammonia in the exit gas stream

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2


Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
4. a gas containing equal parts methane, ethane and ammonia flows at a constant rate through a labor...
Questions


Mathematics, 28.04.2021 02:50







Mathematics, 28.04.2021 02:50

Mathematics, 28.04.2021 02:50

Mathematics, 28.04.2021 02:50


Mathematics, 28.04.2021 02:50

Mathematics, 28.04.2021 02:50

Mathematics, 28.04.2021 02:50

Mathematics, 28.04.2021 02:50

History, 28.04.2021 02:50

French, 28.04.2021 02:50

Mathematics, 28.04.2021 02:50

Mathematics, 28.04.2021 02:50

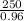 = 260.416 g
= 260.416 g
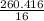 = 16.276 moles
= 16.276 moles
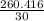 = 8.6805 moles
= 8.6805 moles
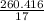 = 15.318 moles
= 15.318 moles
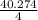 = 40.276 / 4 = 10.068 moles/h
= 40.276 / 4 = 10.068 moles/h
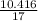 = 0.592 moles
= 0.592 moles