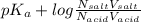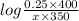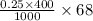Chemistry, 05.10.2019 04:20 Sumitco9578
Calculate how to prepare 750 ml of 0.25 m sodium formate buffer at ph 4. use your textbook to determine the molecular weight and pka of the acid and base. calculate the grams of sodium formate and number of milliliters of formic acid required. then using this stock solution, calculate and describe how you would prepare 100 ml of a 10 mm formate buffer, ph 3.5. by the way, what is the molarity of formic acid?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
Calculate how to prepare 750 ml of 0.25 m sodium formate buffer at ph 4. use your textbook to determ...
Questions


English, 20.03.2020 10:15




Geography, 20.03.2020 10:15








Computers and Technology, 20.03.2020 10:15





History, 20.03.2020 10:15

Mathematics, 20.03.2020 10:15

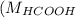 = 46 g/mol
= 46 g/mol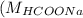 = 68 g/mol
= 68 g/mol of HCOOH = 3.75
of HCOOH = 3.75![[HCOO^{-}]](/tpl/images/0288/1557/bee9e.png) = [HCOOH] + [HCOONa]
= [HCOOH] + [HCOONa]