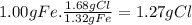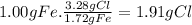Prelab for experiment 4: the atomic theory of matter 1. demonstrate the law of multiple proportions with the following data: a. iron forms two compounds with chlorine, compound x and compound y. when 3.00 g of compound x was analyzed, it was found that 1.68 g of ci combined with 1.32 g of fe. when 5.00 g of compound y was analyzed, it was found that 3.28 g of cl combined with 1.72 g of fe. to illustrate the law, one needs to know how much of one element combines with the same mass as the other element in each compound, but the data above give different masses for both fe and cl. one way to standardize the data is to find out how much of one element, say cl, combines with (if 1.75 g of cl combines with 1.25 g of fe, how many g of cl would combine with 1.00 g of ") do this for both compounds, x and y exactly 1 g of the other, say fe. this can be done by a simple proportion method

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3


Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
Prelab for experiment 4: the atomic theory of matter 1. demonstrate the law of multiple proportions...
Questions


Mathematics, 07.01.2021 04:40

Mathematics, 07.01.2021 04:40



Mathematics, 07.01.2021 04:40

Physics, 07.01.2021 04:50


Mathematics, 07.01.2021 04:50

Arts, 07.01.2021 04:50

Mathematics, 07.01.2021 04:50

Mathematics, 07.01.2021 04:50

English, 07.01.2021 04:50



Computers and Technology, 07.01.2021 04:50



Mathematics, 07.01.2021 04:50

Mathematics, 07.01.2021 04:50