
Chemistry, 06.10.2019 19:30 opgbadwolf5
Ascorbic acid, or vitamin c (c6h8o6, molar mass = 176 g/mol), is a naturally occurring organic compound with antioxidant properties. a healthy adult’s daily requirement of vitamin c is 70-90 mg. a sweet lime contains 2.82×10−4 mol of ascorbic acid.
to determine whether the ascorbic acid in a sweet lime meets the daily requirement, calculate the mass of ascorbic acid in 2.82×10−4 mol of ascorbic acid.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

You know the right answer?
Ascorbic acid, or vitamin c (c6h8o6, molar mass = 176 g/mol), is a naturally occurring organic compo...
Questions


Mathematics, 12.07.2019 08:00


Social Studies, 12.07.2019 08:00


Chemistry, 12.07.2019 08:00




Mathematics, 12.07.2019 08:00

History, 12.07.2019 08:00

Social Studies, 12.07.2019 08:00


Mathematics, 12.07.2019 08:00



History, 12.07.2019 08:00



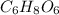 ) contains 6, C atoms 8, H atoms and 6, O atoms .
) contains 6, C atoms 8, H atoms and 6, O atoms .
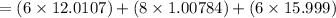
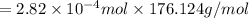
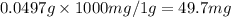 VITAMIN C
VITAMIN C


