
Chemistry, 07.10.2019 00:20 alisonlebron15
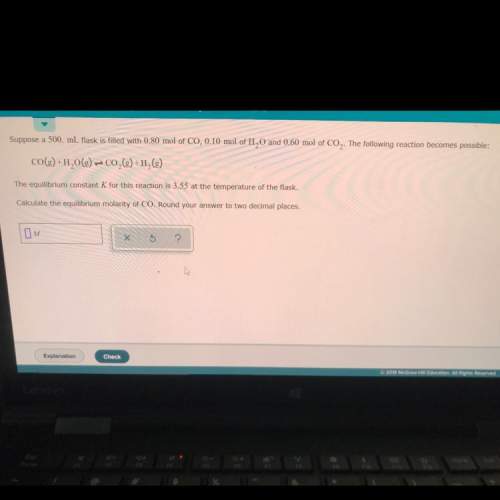
Suppose a 500. ml flask is filled with 0.80 mol of co,0.10 mol of h20 and 0.60 mol of co2. the following reaction becomes possible:
co(g) +h2o(g) + co2(g) +h2(g)
the equilibrium constant k for this reaction is 3.55 at the temperature of the flask.
calculate the equilibrium molarity of co. round your answer to two decimal places.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
The crust of earth may a- continets and ocean floors. b-continents only. c-layers of sedimentary rocks and continents. d-all of the above
Answers: 2

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Suppose a 500. ml flask is filled with 0.80 mol of co,0.10 mol of h20 and 0.60 mol of co2. the follo...
Questions

Mathematics, 15.10.2019 18:30






Computers and Technology, 15.10.2019 18:30

Mathematics, 15.10.2019 18:30



History, 15.10.2019 18:30

Geography, 15.10.2019 18:30


Social Studies, 15.10.2019 18:30


Mathematics, 15.10.2019 18:30


Mathematics, 15.10.2019 18:30

Biology, 15.10.2019 18:30

History, 15.10.2019 18:30



