
Chemistry, 07.10.2019 01:10 sandlobster6274
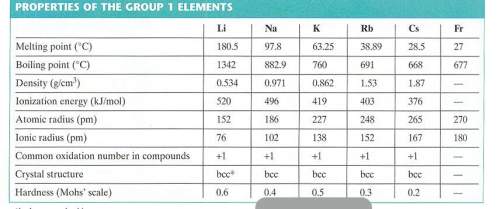
Use the radius of a rubidium atom from the table below to calculate the number of rubidium atoms in a row 6.00 cm long. assume that each rubidium atom touches the ones next to it.
atoms?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1
You know the right answer?
Use the radius of a rubidium atom from the table below to calculate the number of rubidium atoms in...
Questions

Mathematics, 31.03.2020 17:58

English, 31.03.2020 17:58



History, 31.03.2020 17:58



Mathematics, 31.03.2020 17:59

Mathematics, 31.03.2020 17:59



Mathematics, 31.03.2020 17:59

Social Studies, 31.03.2020 17:59



History, 31.03.2020 17:59


Mathematics, 31.03.2020 17:59

History, 31.03.2020 17:59

Mathematics, 31.03.2020 17:59



