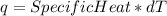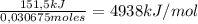Chemistry, 07.10.2019 16:10 dillondelellis2006
Consider the reaction c12h22o11 (s) + 12 o2 (g) → 12 co2 (g) + 11 h2o (l) in which 10.5 g of sucrose, c12h22o11, was burned in a bomb calorimeter with a heat capacity of 7.50 kj/oc (including its water). the temperature inside the calorimeter was found to increase by 20.2 oc. based on this information, what is the heat of this reaction per mole of sucrose?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:10
Amonoprotic acid is an acid that donates a single proton to the solution. suppose you have 0.140 g of a monoprotic acid dissolved in 35.0 ml of water. this solution is then neutralized with 14.5 ml of 0.110 m naoh. what is the molar mass of the acid?
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
Consider the reaction c12h22o11 (s) + 12 o2 (g) → 12 co2 (g) + 11 h2o (l) in which 10.5 g of sucrose...
Questions



English, 15.05.2021 09:40

Mathematics, 15.05.2021 09:40

History, 15.05.2021 09:40

Chemistry, 15.05.2021 09:40

Physics, 15.05.2021 09:40

Computers and Technology, 15.05.2021 09:40

Mathematics, 15.05.2021 09:40

Geography, 15.05.2021 09:40


English, 15.05.2021 09:40

Health, 15.05.2021 09:40

Mathematics, 15.05.2021 09:40

Mathematics, 15.05.2021 09:40

Geography, 15.05.2021 09:40




Advanced Placement (AP), 15.05.2021 09:40