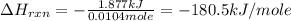Chemistry, 07.10.2019 16:20 chrismeldajbaptiste
You place 33.8 ml of 0.154 m ba(oh)2 in a coffee-cup calorimeter at 25.00°c and add 72.5 ml of 0.648 m hcl, also at 25.00°c. after stirring, the final temperature is 29.22°x. {assume that the total volume is the sum of the individual volumes and that the final solution has the same density (1.00 g/ml) and specific heat capacity (4.184 j/g°c) as water}. calculate the change in enthalpy, \deltaδh, of the reaction (in kj/mol) of water formed. enter the appropriate sign (+/ enter to 1 decimal place.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1
You know the right answer?
You place 33.8 ml of 0.154 m ba(oh)2 in a coffee-cup calorimeter at 25.00°c and add 72.5 ml of 0.648...
Questions


English, 26.02.2021 20:20


Mathematics, 26.02.2021 20:20






Mathematics, 26.02.2021 20:20




Mathematics, 26.02.2021 20:20



Mathematics, 26.02.2021 20:20



Mathematics, 26.02.2021 20:20

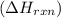 is, -180.5 kJ/mole
is, -180.5 kJ/mole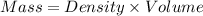
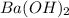
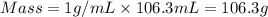
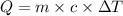
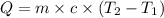
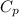 = specific heat capacity of water =
= specific heat capacity of water = 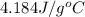
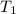 = initial temperature =
= initial temperature = 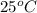
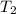 = final temperature =
= final temperature = 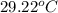
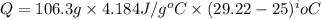
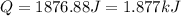 (1 kJ = 1000 J)
(1 kJ = 1000 J)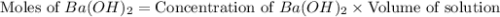
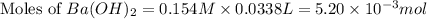
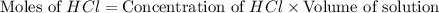
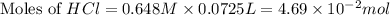
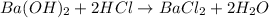
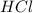
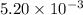 moles of
moles of 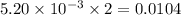 moles of
moles of 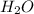
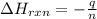
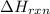 = enthalpy of reaction = ?
= enthalpy of reaction = ?