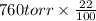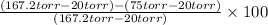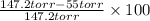Suppose that you are climbing a high mountain and the oxygen partial pressure in the air is reduced to 75 torr. estimate the percentage of the oxygen carrying capacity that will be utilized, assuming that the ph of both tissues and lungs is 7.4 and that the oxygen concentration in the tissues is 20 torr. show all work. i need to know how to work this problem out. the answer is 62.7%. show all work and i will award 5 stars.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 10:30
Ireally need ! calcium metal reacts with a potassium chloride solution to form calcium chloride and potassium ions. balance this reaction. (s) + (aq) → cacl2(s) + +(aq) a) 1, 2, 1, 2 b) 1, 2, 1, 1 c) 1, 1, 1, 1 d) 2, 1, 2, 1
Answers: 1
You know the right answer?
Suppose that you are climbing a high mountain and the oxygen partial pressure in the air is reduced...
Questions






History, 05.11.2019 04:31

Computers and Technology, 05.11.2019 04:31









Computers and Technology, 05.11.2019 04:31

Biology, 05.11.2019 04:31

Mathematics, 05.11.2019 04:31


Computers and Technology, 05.11.2019 04:31

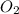 =
=