
Chemistry, 07.10.2019 18:30 oliviaclerk5
Hector claims that a single sodium ion and a single oxygen ion do not bond together to form a stable binary ionic compound. is he correct? why or why not?
a hector is incorrect. the two ions have opposite charges, so they will bond together in a binary ionic compound.
b hector is incorrect. the two ions have more than eighteen protons, so they will bond together in a binary ionic compound.
c hector is correct. the sum of the charges of the two ions must be zero in order for them to form a stable ionic compound.
d hector is correct. the sum of the protons of the two ions must be divisible by eight in order for them to form a stable ionic compound.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3


Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
Hector claims that a single sodium ion and a single oxygen ion do not bond together to form a stable...
Questions






English, 18.05.2021 18:00

Mathematics, 18.05.2021 18:00

Social Studies, 18.05.2021 18:00


History, 18.05.2021 18:00




English, 18.05.2021 18:00

History, 18.05.2021 18:00



English, 18.05.2021 18:00


Physics, 18.05.2021 18:00

![[Na]=1s^22s^22p^63s^1](/tpl/images/0297/6892/4ee3b.png)
![[O]=1s^22s^22p^4](/tpl/images/0297/6892/336ff.png)
![[O^{2-}]=1s^22s^22p^6](/tpl/images/0297/6892/c797e.png)
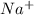 cation and oxide
cation and oxide 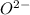 is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral 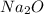 .
.

