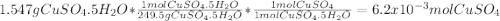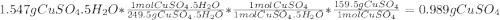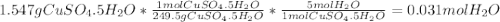Chemistry, 07.10.2019 19:10 jeffro198004
A1.547 g sample of blue copper(ii) sulfate pentahydrate, , is heated carefully to drive off the water. the white crystals of that are left behind have a mass of g. how many moles of were in the original sample? show that the relative molar amounts of and agree with the formula of the hydrate.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
A1.547 g sample of blue copper(ii) sulfate pentahydrate, , is heated carefully to drive off the wa...
Questions

Mathematics, 29.09.2020 14:01

History, 29.09.2020 14:01


Biology, 29.09.2020 14:01





Mathematics, 29.09.2020 14:01


Computers and Technology, 29.09.2020 14:01


Computers and Technology, 29.09.2020 14:01


Spanish, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01


Health, 29.09.2020 14:01

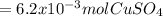
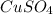 :
: