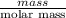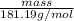Chemistry, 07.10.2019 20:00 mamasmontoya
You do an experiment in which you need 0.5 mole of tyrosine (c9h11no3) .
how many grams must you weigh out?
65 g
90.5 g
181 g
272 g

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1
You know the right answer?
You do an experiment in which you need 0.5 mole of tyrosine (c9h11no3) .
how many grams...
how many grams...
Questions




Computers and Technology, 04.05.2021 19:20

Mathematics, 04.05.2021 19:20




Mathematics, 04.05.2021 19:20

History, 04.05.2021 19:20

Mathematics, 04.05.2021 19:20

Chemistry, 04.05.2021 19:20

History, 04.05.2021 19:20

History, 04.05.2021 19:20






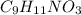 ) is 181.19 g/mol.
) is 181.19 g/mol.