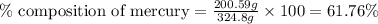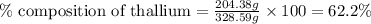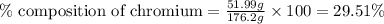Chemistry, 07.10.2019 20:00 hala201490
Be sure to answer all parts. dimercaprol (hsch2chshch2oh) was developed during world war i as an antidote to arsenic-based poison gas and is used today to treat heavy-metal poisoning. it binds the toxic element and carries it out of the body. (a) if each molecule binds one arsenic (as) atom, how many atoms of as could be removed by 696 mg of dimercaprol? × 10 atoms as (enter your answer in scientific notation.) (b) if one molecule binds one metal atom, calculate the mass % of each of the following metals in a metal-dimercaprol combination: mercury, thallium, chromium.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
Be sure to answer all parts. dimercaprol (hsch2chshch2oh) was developed during world war i as an ant...
Questions


Mathematics, 24.11.2021 09:20




Biology, 24.11.2021 09:20

History, 24.11.2021 09:20


Mathematics, 24.11.2021 09:20

Social Studies, 24.11.2021 09:20

English, 24.11.2021 09:20

Mathematics, 24.11.2021 09:20

Mathematics, 24.11.2021 09:20





Mathematics, 24.11.2021 09:30

Mathematics, 24.11.2021 09:30

Mathematics, 24.11.2021 09:30

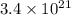
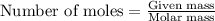
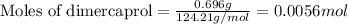
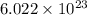 number of molecules.
number of molecules.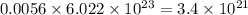 number of molecules.
number of molecules.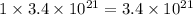 number of arsenic atoms
number of arsenic atoms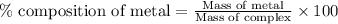 ......(1)
......(1)