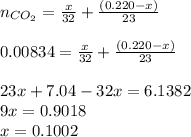An organic liquid is a mixture of methyl alcohol (ch3oh m ch_3oh) and ethyl alcohol (c2h5oh m c_2h_5oh ). a 0.220-g m g sample of the liquid is burned in an excess of o2(g) m o_2(g) and yields 0.367g g co2(g) m co_2(g) (carbon dioxide). set up two algebraic equations, one expressing the mass of carbon dioxide produced in terms of each reagent and the other expressing the mass of sample burned in terms of each reagent.
what is the mass of methyl alcohol (ch3oh m ch_3oh) in the sample?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 1

Chemistry, 23.06.2019 15:30
Sodium chloride can be made as follows: 2na + cl2 ? 2nacl i calculate the maximum amount of nacl possible if 2.3 g of sodium was reacted with excess chlorine. show all your workings.
Answers: 3
You know the right answer?
An organic liquid is a mixture of methyl alcohol (ch3oh m ch_3oh) and ethyl alcohol (c2h5oh m c_2h_5...
Questions




Mathematics, 27.11.2019 23:31


Chemistry, 27.11.2019 23:31

Mathematics, 27.11.2019 23:31

Mathematics, 27.11.2019 23:31



Mathematics, 27.11.2019 23:31


Chemistry, 27.11.2019 23:31

Biology, 27.11.2019 23:31


Mathematics, 27.11.2019 23:31

Mathematics, 27.11.2019 23:31

Business, 27.11.2019 23:31

Health, 27.11.2019 23:31

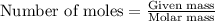 .....(1)
.....(1)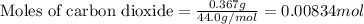
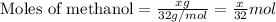
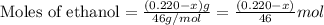
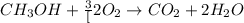
![\frac{x}{32} moles of methanol will produce = [tex]\frac{1}{1}\times \frac{x}{32}=\frac{x}{32}](/tpl/images/0297/8500/66d68.png) moles of carbon dioxide
moles of carbon dioxide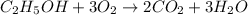
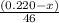 moles of ethanol will produce =
moles of ethanol will produce = 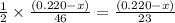 moles of carbon dioxide
moles of carbon dioxide