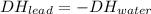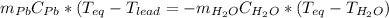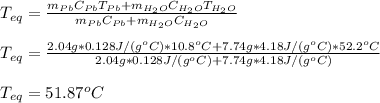Chemistry, 07.10.2019 19:30 amayarayne5
A2.04 g lead weight, initially at 10.8 oc, is submerged in 7.74 g of water at 52.2 oc in an insulated container. clead = 0.128 j/goc; cwater = 4.18 j/goc. what is the final temperature of both the weight and the water at thermal equilibrium?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

You know the right answer?
A2.04 g lead weight, initially at 10.8 oc, is submerged in 7.74 g of water at 52.2 oc in an insulate...
Questions


Chemistry, 09.01.2020 19:31

Spanish, 09.01.2020 19:31




Spanish, 09.01.2020 19:31

Medicine, 09.01.2020 19:31

English, 09.01.2020 19:31

Mathematics, 09.01.2020 19:31

Mathematics, 09.01.2020 19:31


Computers and Technology, 09.01.2020 19:31



Biology, 09.01.2020 19:31

Mathematics, 09.01.2020 19:31

English, 09.01.2020 19:31