
A8.85 g sample of hydrate of sodium carbonate, na, co, xh, o was heated carefully until no more mass was lost from the sample after heating, the final weight of the material was 7.55 g what is the sodium carbonate (na,-water mole ratio?
a) 1: 0
b) 1: 1
c) 2: 1
d) 1: 2

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
A8.85 g sample of hydrate of sodium carbonate, na, co, xh, o was heated carefully until no more mass...
Questions

English, 15.10.2020 03:01

Mathematics, 15.10.2020 03:01




Mathematics, 15.10.2020 03:01

Social Studies, 15.10.2020 03:01

Mathematics, 15.10.2020 03:01


History, 15.10.2020 03:01





Mathematics, 15.10.2020 03:01

Mathematics, 15.10.2020 03:01




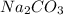 = 105.9888 g/mol
= 105.9888 g/mol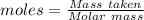
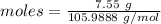
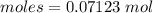
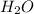 = 18 g/mol
= 18 g/mol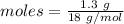
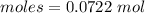
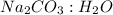 = 0.07123 : 0.0722 = 1 : 1
= 0.07123 : 0.0722 = 1 : 1

