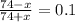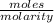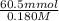Chemistry, 07.10.2019 23:00 crazymadhatter0
Buffer capacity is a measure of a buffer solution's resistance to changes in ph as strong acid or base is added. suppose that you have 185 ml of a buffer that is 0.400 m in both acetic acid (ch3cooh) and its conjugate base (ch3coo−) . calculate the maximum volume of 0.180 m hcl that can be added to the buffer before its buffering capacity is lost.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2


Chemistry, 23.06.2019 06:30
What is the chemical formula for a compound between li and br? libr li2br libr2 libr3
Answers: 1

Chemistry, 23.06.2019 13:30
The two isotopes of chlorine are 3517cl and 3717cl. which isotope is the most abundant?
Answers: 1
You know the right answer?
Buffer capacity is a measure of a buffer solution's resistance to changes in ph as strong acid or ba...
Questions


Mathematics, 27.02.2021 05:20



Mathematics, 27.02.2021 05:20





Mathematics, 27.02.2021 05:20

Mathematics, 27.02.2021 05:20



Mathematics, 27.02.2021 05:20

Business, 27.02.2021 05:20

History, 27.02.2021 05:20

Mathematics, 27.02.2021 05:20

Mathematics, 27.02.2021 05:20


Mathematics, 27.02.2021 05:20

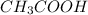 and
and 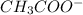 .
.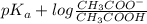
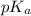 value of acetic acid is 4.76.
value of acetic acid is 4.76. 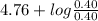
![[CH_{3}COOH]](/tpl/images/0298/3853/d21d8.png) the log term gives a negative value. This means that new pH will be less than 4.76.
the log term gives a negative value. This means that new pH will be less than 4.76.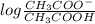
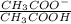 = 0.1 ....... (1)
= 0.1 ....... (1)