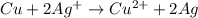Chemistry, 07.10.2019 23:30 icecreamisgood4u
Reaction of copper metal with silver ion. when copper metal is placed in a solution of silver nitrate, a redox reaction forms silver metal and a blue solution of copper(ii) nitrate. why does this solution turn blue?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1


Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Reaction of copper metal with silver ion. when copper metal is placed in a solution of silver nitrat...
Questions

Social Studies, 12.05.2021 14:00


Mathematics, 12.05.2021 14:00

Mathematics, 12.05.2021 14:00


Mathematics, 12.05.2021 14:00

Advanced Placement (AP), 12.05.2021 14:00

Social Studies, 12.05.2021 14:00

History, 12.05.2021 14:00

Mathematics, 12.05.2021 14:00

Mathematics, 12.05.2021 14:00

Mathematics, 12.05.2021 14:00







English, 12.05.2021 14:00

History, 12.05.2021 14:00