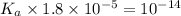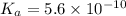Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Given that at 25.0 ∘c ka for hcn is 4.9×10−10 and kb for nh3 is 1.8×10−5, calculate kb for cn− and k...
Questions

English, 03.05.2021 20:00




Mathematics, 03.05.2021 20:00

Biology, 03.05.2021 20:00

Mathematics, 03.05.2021 20:00

Advanced Placement (AP), 03.05.2021 20:00

Mathematics, 03.05.2021 20:00





English, 03.05.2021 20:00


English, 03.05.2021 20:00




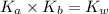
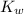 is the dissociation constant of water.
is the dissociation constant of water.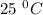 ,
, 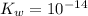
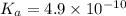
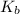 for CN⁻ can be calculated as:
for CN⁻ can be calculated as:
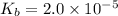
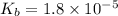
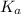 for
for 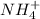 can be calculated as:
can be calculated as: