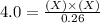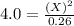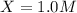Chemistry, 08.10.2019 01:00 hamadehassan
For the equilibrium pcl5(g) pcl3(g) + cl2(g), kc = 4.0 at 228°c. if pure pcl5 is placed in a 1.00-l container and allowed to come to equilibrium, and the equilibrium concentration of pcl5(g) is 0.26 m, what is the equilibrium concentration of pcl3?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
**40** points asapessay questions (10 points possible) clear image of next, create your own scenario. it can be one of your own real experiences or one you make up. use imagery in your writing to give your instructor a the setting and an action taking pace in your writing explain the structure and functions of the skin at work in your scenario. !
Answers: 3

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1
You know the right answer?
For the equilibrium pcl5(g) pcl3(g) + cl2(g), kc = 4.0 at 228°c. if pure pcl5 is placed in a 1.00-l...
Questions


Mathematics, 27.06.2019 08:30


Mathematics, 27.06.2019 08:30


Social Studies, 27.06.2019 08:30

Computers and Technology, 27.06.2019 08:30


Health, 27.06.2019 08:30

History, 27.06.2019 08:30


Mathematics, 27.06.2019 08:30




Mathematics, 27.06.2019 08:30

History, 27.06.2019 08:30



English, 27.06.2019 08:30

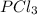 is, 1.0 M
is, 1.0 M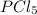 = 0.26 M
= 0.26 M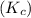 = 4.0
= 4.0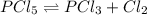
![K_c=\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0298/6790/73fe0.png)
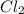 are equal.
are equal.