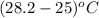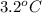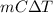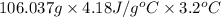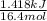Acommon laboratory reaction is the neutralization of an acid with a base. when 32.8 ml of 0.500 m hcl at 25.0°c is added to 66.3 ml of 0.500 m naoh at 25.0°c in a coffee cup calorimeter (with a negligible heat capacity), the temperature of the mixture rises to 28.2°c. what is the heat of reaction per mole of nacl (in kj/mol)? assume the mixture has a specific heat capacity of 4.18 j/(g·k) and that the densities of the reactant solutions are both 1.07 g/ml.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1


Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2
You know the right answer?
Acommon laboratory reaction is the neutralization of an acid with a base. when 32.8 ml of 0.500 m hc...
Questions

Mathematics, 13.06.2021 03:40

Chemistry, 13.06.2021 03:40



Mathematics, 13.06.2021 03:40






Mathematics, 13.06.2021 03:40

History, 13.06.2021 03:40

Mathematics, 13.06.2021 03:40


Mathematics, 13.06.2021 03:40


Mathematics, 13.06.2021 03:40



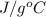

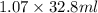
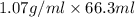
 =
=