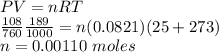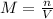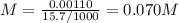The volume of a sample of pure hcl gas was 189 ml at 25°c and 108 mmhg. it was completely dissolved in about 60 ml of water and titrated with an naoh solution; 15.7 ml of the naoh solution were required to neutralize the hcl. calculate the molarity of the naoh solution.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
The volume of a sample of pure hcl gas was 189 ml at 25°c and 108 mmhg. it was completely dissolved...
Questions

Health, 10.10.2019 10:10



Physics, 10.10.2019 10:10



Social Studies, 10.10.2019 10:10


Biology, 10.10.2019 10:10

History, 10.10.2019 10:10

Mathematics, 10.10.2019 10:10

Mathematics, 10.10.2019 10:10


Business, 10.10.2019 10:10

Mathematics, 10.10.2019 10:10


Mathematics, 10.10.2019 10:10


Mathematics, 10.10.2019 10:10