
Chemistry, 08.10.2019 03:30 duncanje5783
Be sure to answer all parts. determine the overall orders of the reactions to which the following rate laws apply: (a) rate = k[no2]2 (b) rate = k zero order first order 1.5 order second order 2.5 order third order zero order first order 1.5 order second order 2.5 order third order (c) rate = k[h2][br2]1/2 (d) rate = k[no2]2[o2] zero order first order 1.5 order second order 2.5 order third order zero order first order 1.5 order second order 2.5 order third order

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1



Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
You know the right answer?
Be sure to answer all parts. determine the overall orders of the reactions to which the following ra...
Questions

Social Studies, 23.08.2019 12:10


Geography, 23.08.2019 12:10

Geography, 23.08.2019 12:10


Chemistry, 23.08.2019 12:10

World Languages, 23.08.2019 12:10



Mathematics, 23.08.2019 12:10

Social Studies, 23.08.2019 12:10


English, 23.08.2019 12:10




History, 23.08.2019 12:10

Biology, 23.08.2019 12:10

Arts, 23.08.2019 12:10

![r=k[NO_2]^2](/tpl/images/0299/0794/8772b.png)
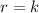
![r=k[H_2]*[Br_2]^{1/2}](/tpl/images/0299/0794/34ab2.png)
![r=k[NO_2]^2[O_2]](/tpl/images/0299/0794/ccd53.png)


