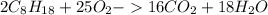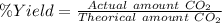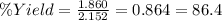Chemistry, 08.10.2019 05:00 justin5647
Using the following equation, determine the % yield from the following reaction if 30.65 g of octane (c_8 8 h_{18} 18 ) react with excess oxygen to produce 81.75 g of co_2 2 (g) 2c_8 8 h_{18} 18 (i) + 25o_2 2 (g) → 16co_2 2 (g) + 18h_2 2 o(l)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1


Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
Using the following equation, determine the % yield from the following reaction if 30.65 g of octane...
Questions





Mathematics, 22.04.2020 03:08

Law, 22.04.2020 03:08

History, 22.04.2020 03:08




History, 22.04.2020 03:08



History, 22.04.2020 03:08

Social Studies, 22.04.2020 03:08