
Hydrogen sulfide (h2s) is a common and troublesome pollutant in industrial wastewaters. one way to remove h2s is to treat the water with chlorine, in which case the following reaction occurs: h2s(aq)+cl2(aq)→s(s)+2h+(aq)+2cl−(a q) the rate of this reaction is first order in each reactant. the rate constant for the disappearance of h2s at 28 ∘c is 3.5×10−2 m−1s−1.if at a given time the concentration of h2s is 2.0×10-4 m and that of cl2 is 2.8×10-2 m , what is the rate of formation of cl?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1
You know the right answer?
Hydrogen sulfide (h2s) is a common and troublesome pollutant in industrial wastewaters. one way to r...
Questions

Mathematics, 10.05.2021 22:10





Mathematics, 10.05.2021 22:10

Mathematics, 10.05.2021 22:10


Chemistry, 10.05.2021 22:10




Mathematics, 10.05.2021 22:10

History, 10.05.2021 22:10


Health, 10.05.2021 22:10

History, 10.05.2021 22:10


Mathematics, 10.05.2021 22:10

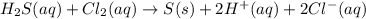
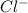 will be twice the rate of disappearance of
will be twice the rate of disappearance of 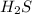 .
.![k[H_{2}S][Cl_{2}]](/tpl/images/0299/3822/2cbd6.png)
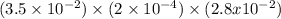
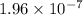 M/s
M/s
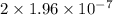
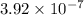 M/s
M/s


