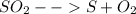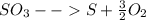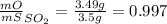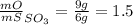Chemistry, 08.10.2019 05:30 aboatright7410
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these were decomposed the sulfur dioxide produced 3.49g oxygen and 3.50g sulfur, while the sulfur trioxide produced 9.00g oxygen and 6.00g sulfur.
a) calculate the mass of oxygen per gram of sulfur for sulfur dioxide.
b) calculate the mass of oxygen per gram of sulfur for sulfur trioxide.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these were decompose...
Questions


Mathematics, 12.11.2020 05:20

World Languages, 12.11.2020 05:20





Mathematics, 12.11.2020 05:20

Computers and Technology, 12.11.2020 05:20


Physics, 12.11.2020 05:20


History, 12.11.2020 05:20



Mathematics, 12.11.2020 05:20


Social Studies, 12.11.2020 05:20


Social Studies, 12.11.2020 05:20