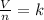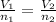flexible container. what will happen if some of

Chemistry, 08.10.2019 19:00 kimjooin02
Aknown number of moles of gas is placed in a
flexible container. what will happen if some of
the gas is removed from the container while the
pressure and temperature are kept constant?
a. the volume will increase.
b. the volume will decrease.
c. the volume will stay the same.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
Aknown number of moles of gas is placed in a
flexible container. what will happen if some of
flexible container. what will happen if some of
Questions


Mathematics, 02.10.2019 00:20




Chemistry, 02.10.2019 00:20







Social Studies, 02.10.2019 00:20



Mathematics, 02.10.2019 00:20




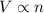 (at constant temperature and pressure)
(at constant temperature and pressure)