
Chemistry, 08.10.2019 21:30 NaVaThEBeAsT
Iron ore can be reduced to iron by the following reaction: fe2o3(s) + 3h2(g) → 2fe + 3h2o(l) (a) how many moles of fe can be made from 1.25 moles of fe2o3? (b) how many moles of h2 are needed to make 3.75 moles of fe? (c) if the reaction yields 12.50 moles of h2o, what mass of fe2o3 was used

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

You know the right answer?
Iron ore can be reduced to iron by the following reaction: fe2o3(s) + 3h2(g) → 2fe + 3h2o(l) (a) ho...
Questions

Biology, 07.10.2019 11:30

Geography, 07.10.2019 11:30

History, 07.10.2019 11:30


Geography, 07.10.2019 11:30


Mathematics, 07.10.2019 11:30


Mathematics, 07.10.2019 11:30

Mathematics, 07.10.2019 11:30



Computers and Technology, 07.10.2019 11:30

Social Studies, 07.10.2019 11:30




Mathematics, 07.10.2019 11:30


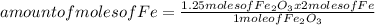
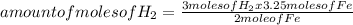
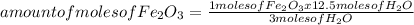
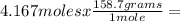 665.4699 grams
665.4699 grams

