
Chemistry, 08.10.2019 23:10 florochoa217
Radioactive decay can be described by the following equation where is the original amount of the substance, is the amount of the substance remaining after time , and is a constant that is characteristic of the substance. for the radioactive isotope iron-55, is if the original amount of iron-55 in a sample is 32.2 mg, how much iron-55 remains after 2.41 years have passed? mg

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
Radioactive decay can be described by the following equation where is the original amount of the sub...
Questions


Mathematics, 25.11.2021 15:40


English, 25.11.2021 15:40




Engineering, 25.11.2021 15:40


Biology, 25.11.2021 15:40

Biology, 25.11.2021 15:40

Chemistry, 25.11.2021 15:40





Arts, 25.11.2021 15:40


Computers and Technology, 25.11.2021 15:40

Mathematics, 25.11.2021 15:40

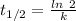
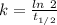
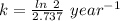
![[A_0]](/tpl/images/0301/7111/9a686.png) = 32.2 mg
= 32.2 mg![[A_t]=[A_0]e^{-kt}](/tpl/images/0301/7111/1ef89.png)
![[A_t]](/tpl/images/0301/7111/5262c.png) is the concentration at time t
is the concentration at time t
![[A_t]=32.2\times e^{-0.2533\times 2.41}\ mg](/tpl/images/0301/7111/46016.png)
![[A_t]=32.2\times e^{-0.610453}\ mg](/tpl/images/0301/7111/d5182.png)
![[A_t]=17.49\ mg](/tpl/images/0301/7111/8a7a9.png)


