
Chemistry, 09.10.2019 01:00 jtaylor4061
The reaction of silver metal and dilute nitric acid proceeds according to the equation above. if 0.10 mole of powdered silver is added to 10.0 milliliters of 6.0 molar nitric acid, the number of moles of no gas that can be formed is
a) 0.015 mole
b) 0.020 mole
c) 0.030 mole
d) 0.045 mole
e) 0.090 mole

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

You know the right answer?
The reaction of silver metal and dilute nitric acid proceeds according to the equation above. if 0.1...
Questions





Mathematics, 10.11.2021 01:00

Mathematics, 10.11.2021 01:00



Mathematics, 10.11.2021 01:00

English, 10.11.2021 01:00

Physics, 10.11.2021 01:00



Mathematics, 10.11.2021 01:00






Mathematics, 10.11.2021 01:00

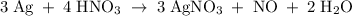
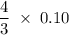 moles nitric acid
moles nitric acid

