
Chemistry, 09.10.2019 02:00 tylerineedhelp
A. the half-life for a first order reaction in 49 minutes. what is the rate constant? b. for a second order reaction, the half-life is 726 seconds. starting with a concentration of 0.600 m, how long will it take for the concentration to decrease to 0.150 m? c. the half-life for a first order reaction is 2768 years. starting with a concentration of 0.345 m, what will the concentration be after 11,072 years? d. the half-life for first order reaction a is 75 minutes. the half-life for a different first order reaction b is 322 minutes. which reaction has the faster rate? (a or b) which reaction has the larger rate constant, k? (a or b)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1

Chemistry, 23.06.2019 13:50
Use the periodic table and your knowledge of isotopes to complete these statements. when polonium-210 emits an alpha particle, the child isotope has an atomic mass of 1-131 undergoes beta-minus decay. the chemical symbol for the new element is fluorine-18 undergoes beta-plus decay. the child isotope has an atomic mass of done intro donne
Answers: 1
You know the right answer?
A. the half-life for a first order reaction in 49 minutes. what is the rate constant? b. for a secon...
Questions




Computers and Technology, 15.02.2022 05:40


English, 15.02.2022 05:40


Computers and Technology, 15.02.2022 05:40


French, 15.02.2022 05:40


Mathematics, 15.02.2022 05:40




Computers and Technology, 15.02.2022 05:40

Social Studies, 15.02.2022 05:40



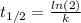 (1) As half.life is 49 min. Rate constant -k- is: 0,014 min⁻¹
(1) As half.life is 49 min. Rate constant -k- is: 0,014 min⁻¹

