
Chemistry, 09.10.2019 02:20 savannah19dw
Consider the reaction a + b → products from the following data obtained at a certain temperature, determine the order of the reaction. enter the order with respect to a, the order with respect to b, and the overall reaction order. a [a] (m) [b] (m) rate (m/s) 1.50 1.50 3.20 × 10−1 1.50 2.50 3.20 × 10−1 3.00 1.50 6.40 × 10−1 b reaction

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1
You know the right answer?
Consider the reaction a + b → products from the following data obtained at a certain temperature, de...
Questions

Mathematics, 19.10.2019 10:30




History, 19.10.2019 10:30

English, 19.10.2019 10:30


Mathematics, 19.10.2019 10:30

Mathematics, 19.10.2019 10:30

Physics, 19.10.2019 10:30

Mathematics, 19.10.2019 10:30



Mathematics, 19.10.2019 10:30

History, 19.10.2019 10:30


Mathematics, 19.10.2019 10:30

World Languages, 19.10.2019 10:30

Mathematics, 19.10.2019 10:30

Social Studies, 19.10.2019 10:30

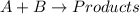
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0302/2044/10aeb.png)
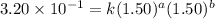 ....(1)
....(1)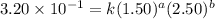 ....(2)
....(2)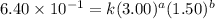 ....(3)
....(3)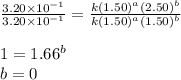
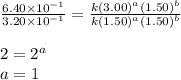
![\text{Rate}=k[A]^1[B]^0](/tpl/images/0302/2044/de6a4.png)
![\text{Rate}=k[A]](/tpl/images/0302/2044/660c6.png)


