
Chemistry, 09.10.2019 03:00 destiny465
H2so4 is an unusual diprotic acid in that the first dissociation is considered strong, but the second dissociation is weak. therefore, h2so4 only has ka2 = 1.2x10−2. consider an aqueous solution containing 0.010 m h2so4. calculate the concentration of so42− ions in this solution. calculate the concentration of hso4− ions in this solution. calculate the concentration of h3o+ ions the solution. enter each of your answers to two significant figures. do not use scientific notation.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
H2so4 is an unusual diprotic acid in that the first dissociation is considered strong, but the secon...
Questions

Mathematics, 10.02.2022 18:00


History, 10.02.2022 18:00




Mathematics, 10.02.2022 18:00

Law, 10.02.2022 18:00


English, 10.02.2022 18:10

Mathematics, 10.02.2022 18:10


Biology, 10.02.2022 18:10

History, 10.02.2022 18:10

Mathematics, 10.02.2022 18:10


English, 10.02.2022 18:10


Physics, 10.02.2022 18:10

Mathematics, 10.02.2022 18:10

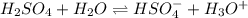
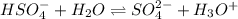
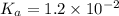 . Hence, formula for
. Hence, formula for 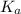 is as follows.
is as follows.![K_{a} = \frac{[SO^{2-}_{4}][H_{3}O^{+}]}{[HSO^{-}_{4}]}](/tpl/images/0302/2941/4c298.png)
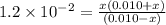
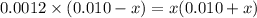
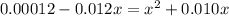
![[SO^{2-}_{4}]](/tpl/images/0302/2941/a4a68.png) = x,
= x, ![[HSO^{-}_{4}]](/tpl/images/0302/2941/b5b89.png) = 0.010 - x
= 0.010 - x![[H_{3}O^{+}]](/tpl/images/0302/2941/71b58.png) = 0.010 + x
= 0.010 + x


