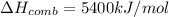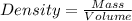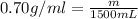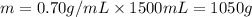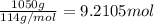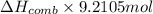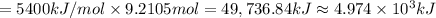The automobile fuel called e85 consists of 85 % ethanol and 15 % gasoline. e85 can be used in so-called "flex-fuel" vehicles (ffvs), which can use gasoline, ethanol, or a mix as fuels. assume that gasoline consists of a mixture of octanes (different isomers of c8h18), that the average heat of combustion of c8h18(l) is 5400 kj/mol, and that gasoline has an average density of 0.70 g/ml. the density of ethanol is 0.79 g/ml. by using the information given, calculate the energy produced by combustion of 1.5 l of gasoline.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 2
You know the right answer?
The automobile fuel called e85 consists of 85 % ethanol and 15 % gasoline. e85 can be used in so-cal...
Questions



Mathematics, 05.01.2021 17:10

History, 05.01.2021 17:10

Biology, 05.01.2021 17:10


Mathematics, 05.01.2021 17:10

Mathematics, 05.01.2021 17:10


French, 05.01.2021 17:10

English, 05.01.2021 17:10


English, 05.01.2021 17:10



Mathematics, 05.01.2021 17:10

English, 05.01.2021 17:10


Mathematics, 05.01.2021 17:10

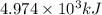 of energy is produced by combustion of 1.5 L of gasoline.
of energy is produced by combustion of 1.5 L of gasoline.