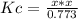Chemistry, 09.10.2019 05:00 katiedurden02
Phosgene is a potent chemical warfare agent that is now outlawed by international agreement. it decomposes by the reaction: cocl2 (g) ⇋ co (g) + cl2 (g) kc = 7.5 x 10-5 (at 362°c) calculate the concentration of co when 7.73 mol of phosgene decomposes and reaches equilibrium in a 10.0 liter flask. multiply your answer by 103 and enter that number to 2 decimal places. hint: look at sample problem 17.9 in the 8th ed of silberberg. write a kc expression. calculate the initial concentration of phosgene. do an ice chart. find the concentration of co.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
Phosgene is a potent chemical warfare agent that is now outlawed by international agreement. it deco...
Questions


Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00

English, 10.03.2021 01:00



English, 10.03.2021 01:00




Mathematics, 10.03.2021 01:00

Mathematics, 10.03.2021 01:00




Geography, 10.03.2021 01:00


![Kc = \frac{[C]^cx[D]^d}{[A]^ax[B]^b}](/tpl/images/0302/6261/d3f86.png)