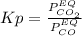Chemistry, 09.10.2019 05:30 minnahelhoor
Nickel (ii) oxide reacts with carbon monoxide to form nickel metal. co(g) + nio(s) ⇄ co2(g) + ni(s) kp = 20 at 873 k if a reaction vessel at equilibrium contains solid ni, solid nio, 400 mm hg of co2, and 20 mm hg of co, doubling the amount of co(g) present will lead to the production of more solid nickel at 873 k. group of answer choices

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2


Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
Nickel (ii) oxide reacts with carbon monoxide to form nickel metal. co(g) + nio(s) ⇄ co2(g) + ni(s)...
Questions


Spanish, 22.06.2019 09:00

History, 22.06.2019 09:00


Social Studies, 22.06.2019 09:00


History, 22.06.2019 09:00


Biology, 22.06.2019 09:00



Mathematics, 22.06.2019 09:00

Arts, 22.06.2019 09:00




Geography, 22.06.2019 09:00