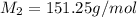Chemistry, 09.10.2019 18:00 Derienw6586
Benzyl acetate is one of the active components of oil of jasmine. if 0.125 g of the compound is added to 25.0g of chloroform (chcl 3 ), the boiling point of the solution is 61.82°c. what is the molar mass of benzyl acetate. (chloroform: kb = 3.63 °c/m; tb° 61.70°c)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Use examples from the article to explain one positive and one negative effect that chemistry has had on society
Answers: 2

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
Benzyl acetate is one of the active components of oil of jasmine. if 0.125 g of the compound is adde...
Questions


Biology, 26.11.2020 01:10




Chemistry, 26.11.2020 01:10

Mathematics, 26.11.2020 01:10

Mathematics, 26.11.2020 01:10

Health, 26.11.2020 01:10




Mathematics, 26.11.2020 01:10

Mathematics, 26.11.2020 01:10


Computers and Technology, 26.11.2020 01:10



Mathematics, 26.11.2020 01:20

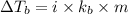
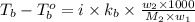
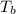 = boiling point of solution =
= boiling point of solution = 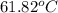
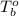 = boiling point of chloroform =
= boiling point of chloroform = 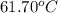
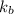 = boiling point constant of chloroform =
= boiling point constant of chloroform = 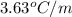
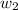 = mass of solute (Benzyl acetate) = 0.125 g
= mass of solute (Benzyl acetate) = 0.125 g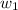 = mass of solvent (chloroform) = 25.0 g
= mass of solvent (chloroform) = 25.0 g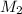 = molar mass of solute (Benzyl acetate) = ?
= molar mass of solute (Benzyl acetate) = ?