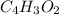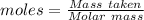Terephthalic acid is an important chemical used in the manufacture of polyesters and plasticizers. it contains only , , and . combustion of 50.20 mg terephthalic acid produces 106.4 mg and 16.34 mg . if 0.170 mole of terephthalic acid has a mass of 28.2 g, determine the molecular formula for terephthalic acid.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
Terephthalic acid is an important chemical used in the manufacture of polyesters and plasticizers. i...
Questions


Mathematics, 09.02.2021 20:10

Mathematics, 09.02.2021 20:10


Arts, 09.02.2021 20:10

Mathematics, 09.02.2021 20:10

Mathematics, 09.02.2021 20:10


Mathematics, 09.02.2021 20:10


English, 09.02.2021 20:10



Social Studies, 09.02.2021 20:10

Mathematics, 09.02.2021 20:10


Computers and Technology, 09.02.2021 20:10



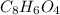
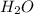 = 16.34 ×10⁻³ g /18 g/mol = 0.000907778 moles
= 16.34 ×10⁻³ g /18 g/mol = 0.000907778 moles
 = 106.4 ×10⁻³ g /44.01 g/mol = 0.002418 moles
= 106.4 ×10⁻³ g /44.01 g/mol = 0.002418 moles