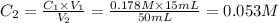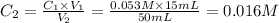Achemist places 2.5316 g of na 2so 4 in a 100 ml volumetric flask and adds water to the mark. she then pipets 15 ml of the resulting solution into a 50 ml volumetric flask and adds water to the mark and mixes to make a solution. she then pipets 15 ml of this new solution into a 50 ml volumetric flask and dilutes to the mark. determine the molar concentration of sodium sulfate in the most dilute solution prepared.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

You know the right answer?
Achemist places 2.5316 g of na 2so 4 in a 100 ml volumetric flask and adds water to the mark. she th...
Questions

Mathematics, 04.12.2019 18:31


Mathematics, 04.12.2019 18:31

Biology, 04.12.2019 18:31

Chemistry, 04.12.2019 18:31







Computers and Technology, 04.12.2019 18:31

History, 04.12.2019 18:31

History, 04.12.2019 18:31


English, 04.12.2019 18:31

Mathematics, 04.12.2019 18:31

Mathematics, 04.12.2019 18:31

Spanish, 04.12.2019 18:31

![[Na_{2}SO_{4}]=\frac{moles(Na_{2}SO_{4})}{liters(solution)} =\frac{mass((Na_{2}SO_{4}))}{molarmass(moles(Na_{2}SO_{4}) \times 0.100L)} =\frac{2.5316g}{142g/mol\times 0.100L } =0.178M](/tpl/images/0304/7678/6942e.png)