
For the following reaction, kc = 15 at 700 k. 2 no(g) + cl2(g) ⇄ 2 nocl(g) if we have [no] = 0.15 m, [cl2] = 0.15 m, [nocl] = 0.40 m at 700 k, what will happen? group of answer choices the equilibrium will not shift. the equilibrium will shift to the left, but will use up only part of the nocl. the equilibrium will shift to the right, but will use up only part of the no and cl2. the equilibrium will shift to the right until all the reactants are used up. the equilibrium will shift to the left until all the nocl is used up.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2
You know the right answer?
For the following reaction, kc = 15 at 700 k. 2 no(g) + cl2(g) ⇄ 2 nocl(g) if we have [no] = 0.15 m,...
Questions

Business, 02.09.2021 23:50

Biology, 02.09.2021 23:50

Biology, 02.09.2021 23:50

History, 02.09.2021 23:50







English, 02.09.2021 23:50

Spanish, 02.09.2021 23:50

Mathematics, 02.09.2021 23:50





Mathematics, 02.09.2021 23:50

Mathematics, 02.09.2021 23:50

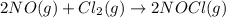
![Q=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0305/1218/afbe9.png)
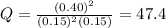
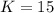
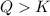 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.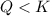 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.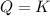 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.

