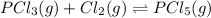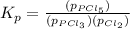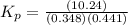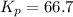Chemistry, 10.10.2019 00:10 GingerSnaps
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to the reaction: pcl3 (g) + cl2 (g) → pcl5 (g) an equilibrium mixture at 450 k contains ppcl3 = 0.348 atm, pcl2 = 0.441 atm, and ppcl5 = 10.24 atm. what is the value of kp at this temperature? phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to the reaction: (g) + (g) (g) an equilibrium mixture at 450 k contains = 0.348 atm, = 0.441 atm, and = 10.24 atm. what is the value of kp at this temperature? 66.7 1.50 ⋅ 10−2 12.99 1.57 9.45

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

You know the right answer?
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlor...
Questions

Mathematics, 05.10.2019 19:40

Health, 05.10.2019 19:40


Social Studies, 05.10.2019 19:40



Mathematics, 05.10.2019 19:40




History, 05.10.2019 19:40

Mathematics, 05.10.2019 19:40


Social Studies, 05.10.2019 19:40


Mathematics, 05.10.2019 19:40


Chemistry, 05.10.2019 19:40

Mathematics, 05.10.2019 19:40

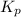 at this temperature is 66.7
at this temperature is 66.7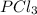 at equilibrium = 0.348 atm
at equilibrium = 0.348 atm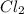 at equilibrium = 0.441 atm
at equilibrium = 0.441 atm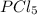 at equilibrium = 10.24 atm
at equilibrium = 10.24 atm